usp class vi compliant
The USP Class VI compounds must be. RoHS a European Union Directive restricts the use of.

Double Bagged Usp Class Vi Liquid Funnels
For tubing with at least 0001 wall thickness and OD of 0012500770.

. Sil 714001 USP class VI Silicone 1 70 Yes transl. A new USP Class VI-compliant substrate for manufacturing disposable microfluidic devices. USP Class VI Approved Plastic Materials.
While it is possible a USP Class VI material could also be ISO 10993 compliant its not a given and USP Class VI alone is not sufficient for adherence to ISO 10993. Some medical silicones must meet USP Class VI FDA CFR 21 1772600 and RoHS requirements. EPDM red Silicone and Viton.
Contact us to discuss cutting other tubing. Valves restrict flow when fitting halves are disconnected. ISO 90012015 Certified QMS.
Overview of USP Class VI Approved Plastic Materials. What is ADI-Free BSE-Free TSE-Free. High quality USP Class VI compliant sheet material made from our own specially formulated compounds.
ADI-free certifies that the raw materials. Fitting body constructed of durable. Class VI testing is aimed to certify that there are no harmful reactions or long-term bodily effects caused by chemicals that leach out of plastic materials.
Theres even a silicon carbide surface thats compliant and various release agents. USP Class Testing standards are determined by the United States. USP Class testing is one of the most common methods of testing to determine bio-compatibility of materials.
Sheet material is available in various thick-nesses. There are plenty of silicone products and other medical grade plastics that are USP Class VI compliant. Sil 714002 USP class VI Silicone 1 70 Yes transl.
Enflo products are USP Class VI FDA ROHS REACH and Conflict Materials compliant. ENFLON is a registered trademark for Enflos filled PTFE. Compliance to USP Class VI is often requested by users in the biopharmaceutical and medical industries.
Kuoa Laiying Nga Gloria S. There are six classes VI being the most rigorous. Pharmacopoeia Class VI judges the suitability of plastic material intended for use.
Testing for biocompatibility of materials is mainly centered on guidelines put forth by ISO 10993 the FDA United States Regulations EU 2017745-6 European Union and the USP. Pharmacopoeia USP Class VI outlines requirements for system toxicity and intracutaneous toxicity for these cleaner compounds. Moulded O-rings class 1 less than 10 furnace black These can be produced in all.
Has a full range of specialty adhesives epoxies primers for polyolefins UV curables and silicones that have been fully tested to meet USP Class VI. Body and seal materials are made of USP Class VI-compliant and BSETSE-free materials.

O Rings Fda And Usp Class Vi Darcoid Rubber Company Oakland California
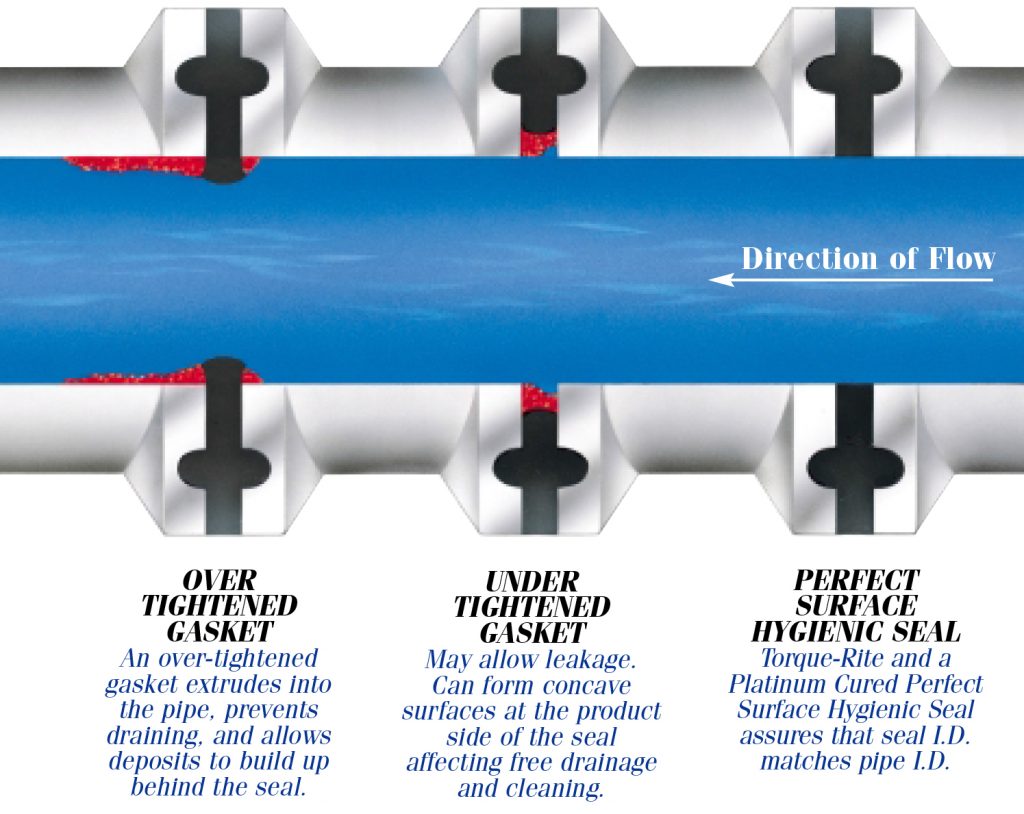
Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa

Usp Class Vi Foster Corporation

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing

Usp Class Vi Gaskets Seals Usp Class 6 O Rings Ppe

Why You Need Certified Usp Class Vi Silicones Specialty Silicone Products Inc

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

Rulon 641 Usp Approved And Fda Compliant Tristar Plastics

Fda Usda Nsf51 Usp Class Vi Compliant Seals Products

Biocompatic Usp Class Vi Silicone Cable Alternative Northwire Inc

Is Dursan Usp Class Vi Compliant